A medication used to support fertility by stimulating the development and ripening of ovarian follicles and triggering ovulation.
Indications:
- Development and maturation of multiple follicles in assisted reproductive technologies, such as in vitro fertilization (IVF).
- Ovulation induction in women with anovulation or oligo-ovulation.
- Support in fertility treatments requiring controlled ovarian stimulation.
Main Active Ingredients:
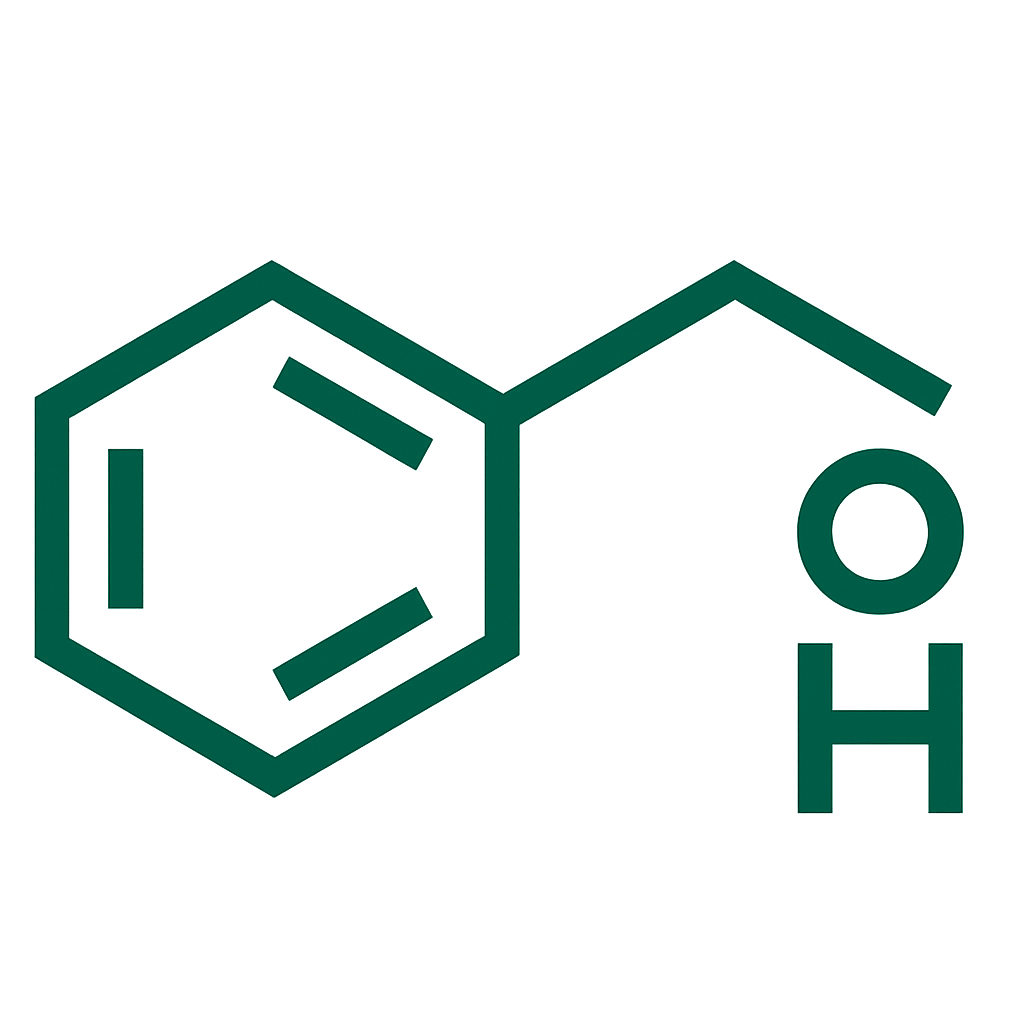
- Choriogonadotropin Alfa 250 mcg/0.5 mL (equivalent to 6500 IU).
Precautions:
- Do not use if allergic to choriogonadotropin alfa or any other ingredients.
- Avoid if you have ovarian cysts of unknown origin, tumors of reproductive organs, severe venous thromboembolic disorders, or conditions preventing normal pregnancy.
- Fertility evaluation of you and your partner is recommended before treatment.
- Closely monitor for ovarian hyperstimulation syndrome (OHSS) and multiple pregnancy.
- Consult a doctor if you have a history of thromboembolic events, ectopic pregnancy, or miscarriage.
- Not for use in children, adolescents, pregnant, or breastfeeding women.
- Do not exceed the recommended dose.
Side Effects:
- Headache.
- Injection site reactions (pain, redness, swelling).
- Diarrhea.
- Allergic reactions.
- OHSS (abdominal pain, nausea, vomiting, rapid weight gain).
- Severe OHSS with fluid accumulation, thromboembolic events.
Drug-Drug Interactions:
- Inform your doctor about any current or recent medications, as interactions may occur.
Choriogonadotropin Alfa, Mannitol, Methionine, Poloxamer 188, Phosphoric Acid, Sodium Hydroxide, Water For Injections.