Cinfa
SKU: 48751
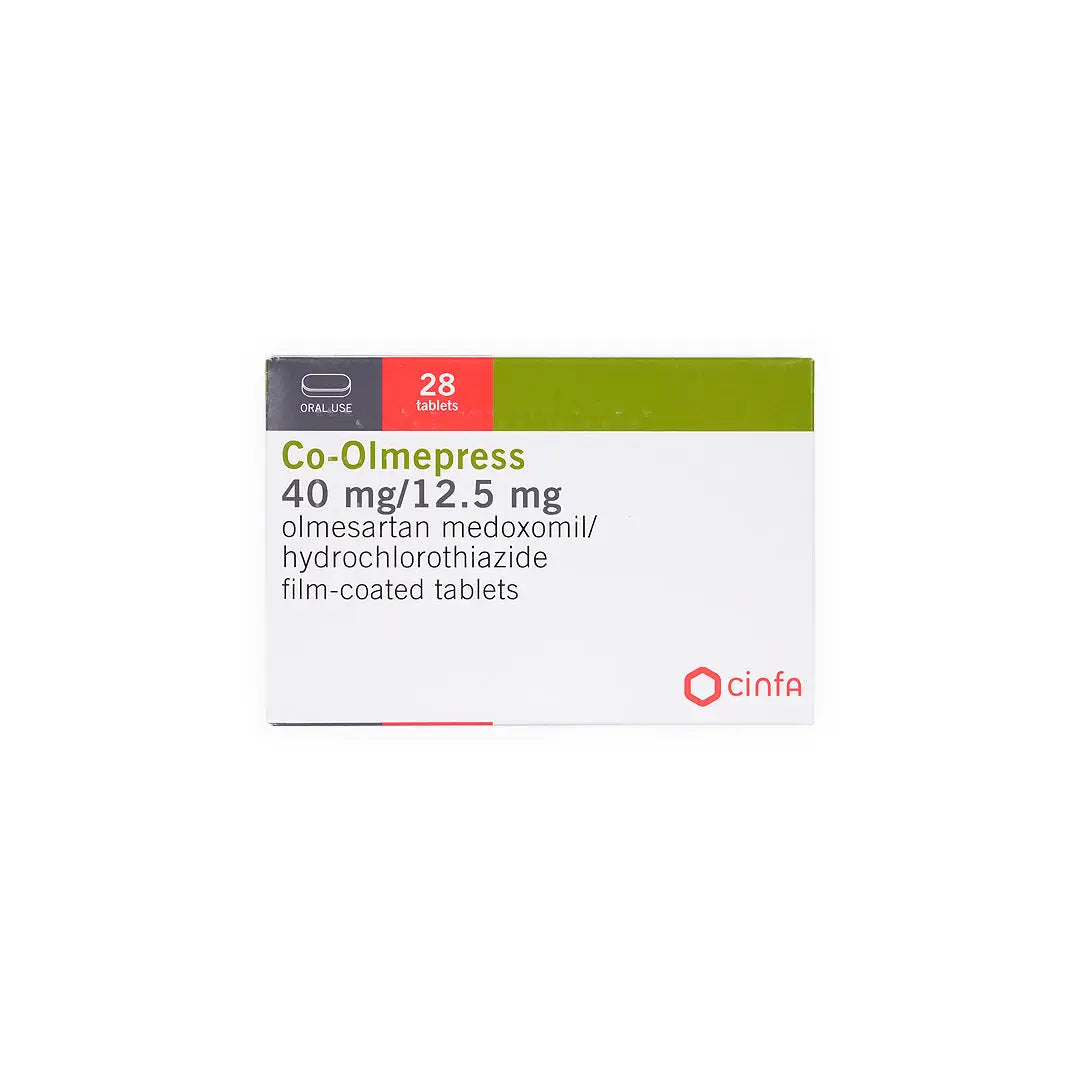
A combination antihypertensive tablet that merges Olmesartan medoxomil with Hydrochlorothiazide, enabling effective blood pressure control when monotherapy with an angiotensin receptor blocker is insufficient.
Indications:
Treatment of essential hypertension not well-controlled by Olmesartan alone.
Main Active Ingredients:
- Olmesartan medoxomil.
- Hydrochlorothiazide.
Precautions:
- Contraindicated in patients with hypersensitivity to any component, severe hepatic or renal impairment, or anuria, and during the 2nd & 3rd trimesters of pregnancy.
- Use caution and monitor blood pressure and kidney function in patients who are volume or salt-depleted, or have renal artery stenosis.
- May precipitate electrolyte imbalances, monitor serum creatinine, potassium, sodium, and uric acid levels.
Side Effects:
- Dizziness, headache, fatigue, gastrointestinal symptoms (e.g., nausea, stomach discomfort), cough, urinary or respiratory tract infections, and musculoskeletal pain.
- Elevated uric acid, potassium, glucose, liver enzymes, blood urea, creatinine; decreased sodium, chloride, magnesium; and altered platelet counts.
Drug–Drug Interactions:
- Other antihypertensives, NSAIDs, diuretics, antiarrhythmics, steroids, antidiabetics, anticoagulants (e.g., warfarin), cardiac glycosides (e.g., digoxin), and lipid-lowering agents.
- Aliskiren in diabetic patients or those with renal impairment (GFR <60 mL/min/1.73 m²).
Indications:
Treatment of essential hypertension not well-controlled by Olmesartan alone.
Main Active Ingredients:
- Olmesartan medoxomil.
- Hydrochlorothiazide.
Precautions:
- Contraindicated in patients with hypersensitivity to any component, severe hepatic or renal impairment, or anuria, and during the 2nd & 3rd trimesters of pregnancy.
- Use caution and monitor blood pressure and kidney function in patients who are volume or salt-depleted, or have renal artery stenosis.
- May precipitate electrolyte imbalances, monitor serum creatinine, potassium, sodium, and uric acid levels.
Side Effects:
- Dizziness, headache, fatigue, gastrointestinal symptoms (e.g., nausea, stomach discomfort), cough, urinary or respiratory tract infections, and musculoskeletal pain.
- Elevated uric acid, potassium, glucose, liver enzymes, blood urea, creatinine; decreased sodium, chloride, magnesium; and altered platelet counts.
Drug–Drug Interactions:
- Other antihypertensives, NSAIDs, diuretics, antiarrhythmics, steroids, antidiabetics, anticoagulants (e.g., warfarin), cardiac glycosides (e.g., digoxin), and lipid-lowering agents.
- Aliskiren in diabetic patients or those with renal impairment (GFR <60 mL/min/1.73 m²).
Olmesartan medoxomil, Hydrochlorothiazide, Microcrystalline cellulose, Lactose monohydrate, Low-substituted hydroxypropyl cellulose, Hypromellose, Magnesium stearate, Titanium dioxide (E171), Talc, Iron oxide yellow (E172).
Recently Viewed