GSK
SKU: 10399
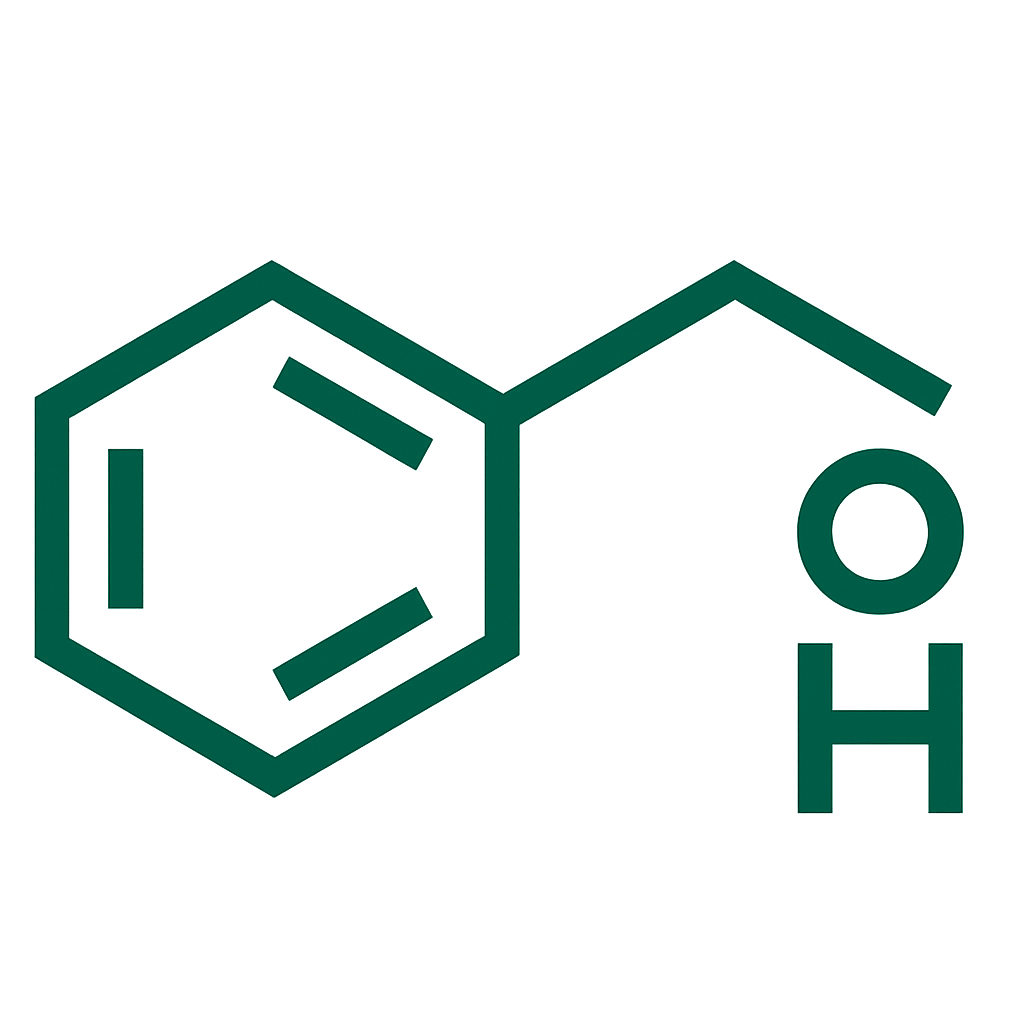
An antiepileptic medication containing levetiracetam, used to help control seizures in various types of epilepsy. It works by modulating neurotransmitter release through binding to synaptic vesicle protein SV2A, stabilizing electrical activity in the brain.
Indications:
- Adjunctive therapy in partial-onset seizures (with or without secondary generalization) in adults and children.
- Monotherapy in newly diagnosed partial seizures in adults and adolescents.
- Adjunctive treatment of myoclonic seizures in adults and adolescents with juvenile myoclonic epilepsy.
- Adjunctive treatment of primary generalized tonic-clonic seizures in adults and children with idiopathic generalized epilepsy.
Main Active Ingredient:
- Levetiracetam 1000 mg per tablet
Precautions:
- Not for use in patients with known hypersensitivity to levetiracetam or any excipient.
- Use cautiously in patients with renal impairment (dose adjustment required).
- Monitor for behavioral changes (aggression, irritability, mood swings, depression, suicidal thoughts).
- Avoid abrupt discontinuation; taper gradually to reduce seizure risk.
- Safety during pregnancy and breastfeeding requires medical advice.
- Caution in elderly patients due to renal clearance reduction.
Possible Side Effects:
- Somnolence.
- fatigue.
- Dizziness.
- Headache.
- Nasopharyngitis.
- Irritability.
- Depression.
- Anxiety.
- Aggression.
insomnia.
- Nausea.
- Vomiting.
- Diarrhea.
- Abdominal pain.
- Suicidal ideation.
- Psychosis.
- Hallucinations.
- Severe allergic reactions.
- Pancytopenia.
- Severe skin reactions (StevensJohnson syndrome).
- Hepatic dysfunction.
Drug-Drug Interactions:
- Enzyme inducers/inhibitors generally do not significantly affect levels.
- Alcohol.
- Co-administration with CNS depressants.
- Probenecid.
Indications:
- Adjunctive therapy in partial-onset seizures (with or without secondary generalization) in adults and children.
- Monotherapy in newly diagnosed partial seizures in adults and adolescents.
- Adjunctive treatment of myoclonic seizures in adults and adolescents with juvenile myoclonic epilepsy.
- Adjunctive treatment of primary generalized tonic-clonic seizures in adults and children with idiopathic generalized epilepsy.
Main Active Ingredient:
- Levetiracetam 1000 mg per tablet
Precautions:
- Not for use in patients with known hypersensitivity to levetiracetam or any excipient.
- Use cautiously in patients with renal impairment (dose adjustment required).
- Monitor for behavioral changes (aggression, irritability, mood swings, depression, suicidal thoughts).
- Avoid abrupt discontinuation; taper gradually to reduce seizure risk.
- Safety during pregnancy and breastfeeding requires medical advice.
- Caution in elderly patients due to renal clearance reduction.
Possible Side Effects:
- Somnolence.
- fatigue.
- Dizziness.
- Headache.
- Nasopharyngitis.
- Irritability.
- Depression.
- Anxiety.
- Aggression.
insomnia.
- Nausea.
- Vomiting.
- Diarrhea.
- Abdominal pain.
- Suicidal ideation.
- Psychosis.
- Hallucinations.
- Severe allergic reactions.
- Pancytopenia.
- Severe skin reactions (StevensJohnson syndrome).
- Hepatic dysfunction.
Drug-Drug Interactions:
- Enzyme inducers/inhibitors generally do not significantly affect levels.
- Alcohol.
- Co-administration with CNS depressants.
- Probenecid.
Levetiracetam,Corn starch,Povidone K30,Colloidal anhydrous silica,Magnesium stearate,Hypromellose,Titanium dioxide,Macrogol 400,Iron oxide black.
Recently Viewed