Bayer
SKU: 13060
A levonorgestrel-releasing intrauterine system (IUS) that provides long-term reversible contraception for up to 5 years. It works by thickening cervical mucus, inhibiting sperm motility and function, and thinning the endometrium, thereby preventing fertilization and implantation.
Indications:
- Long-term contraception (up to 5 years).
- Treatment of heavy menstrual bleeding (menorrhagia) in women choosing intrauterine contraception.
- Endometrial protection during estrogen replacement therapy (in postmenopausal women).
Main Active Ingredient:
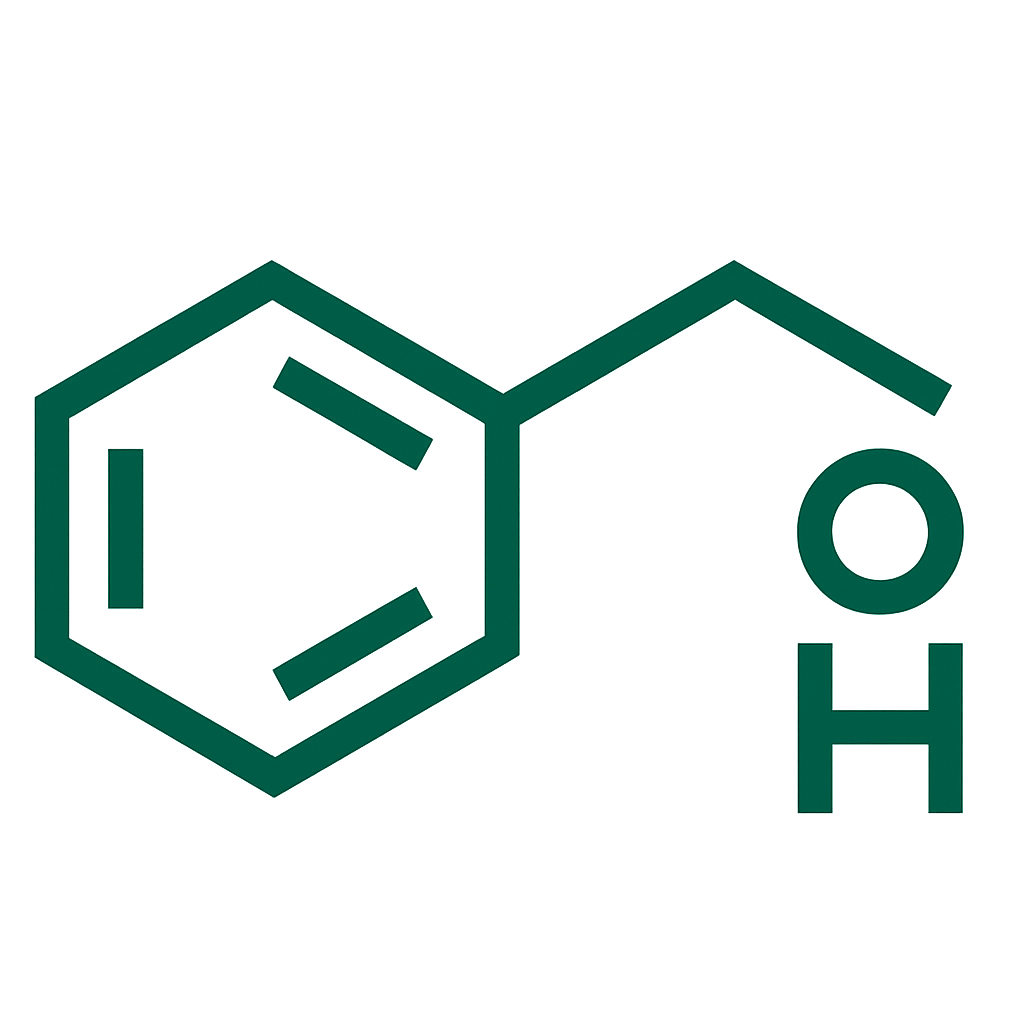
- Levonorgestrel (20 micrograms released per 24 hours initially, gradually decreasing over time).
Precautions:
Contraindicated in:
- Pregnancy or suspected pregnancy.
- Acute pelvic inflammatory disease (PID) or history of recurrent PID.
- Uterine abnormalities (congenital or acquired) that distort the uterine cavity.
- Known or suspected breast cancer, uterine or cervical malignancy.
- Unexplained vaginal bleeding.
- Active liver disease or liver tumor.
Use with caution in:
- Women with increased risk of ectopic pregnancy.
- History of migraine or severe headache.
- Hypertension or cardiovascular disease.
- Diabetes (monitor glucose tolerance).
- Regular medical checkups and self-check for presence of IUD threads are advised.
Side Effects:
- Irregular bleeding or spotting.
- Amenorrhea.
- Ovarian cysts.
- Breast tenderness.
- Abdominal pain.
- Acne.
- Mood changes.
- Headache.
- Decreased libido.
- Back pain.
- Expulsion of the device.
- Uterine perforation.
- Pelvic infection.
- Hypersensitivity reactions.
Drug-Drug Interactions:
- Enzyme-inducing drugs may theoretically reduce levonorgestrel effectiveness.
- Corticosteroids and certain immunosuppressants may increase infection risk.
- Interaction with other hormonal contraceptives if used simultaneously.
Indications:
- Long-term contraception (up to 5 years).
- Treatment of heavy menstrual bleeding (menorrhagia) in women choosing intrauterine contraception.
- Endometrial protection during estrogen replacement therapy (in postmenopausal women).
Main Active Ingredient:
- Levonorgestrel (20 micrograms released per 24 hours initially, gradually decreasing over time).
Precautions:
Contraindicated in:
- Pregnancy or suspected pregnancy.
- Acute pelvic inflammatory disease (PID) or history of recurrent PID.
- Uterine abnormalities (congenital or acquired) that distort the uterine cavity.
- Known or suspected breast cancer, uterine or cervical malignancy.
- Unexplained vaginal bleeding.
- Active liver disease or liver tumor.
Use with caution in:
- Women with increased risk of ectopic pregnancy.
- History of migraine or severe headache.
- Hypertension or cardiovascular disease.
- Diabetes (monitor glucose tolerance).
- Regular medical checkups and self-check for presence of IUD threads are advised.
Side Effects:
- Irregular bleeding or spotting.
- Amenorrhea.
- Ovarian cysts.
- Breast tenderness.
- Abdominal pain.
- Acne.
- Mood changes.
- Headache.
- Decreased libido.
- Back pain.
- Expulsion of the device.
- Uterine perforation.
- Pelvic infection.
- Hypersensitivity reactions.
Drug-Drug Interactions:
- Enzyme-inducing drugs may theoretically reduce levonorgestrel effectiveness.
- Corticosteroids and certain immunosuppressants may increase infection risk.
- Interaction with other hormonal contraceptives if used simultaneously.
Levonorgestrel,Polydimethylsiloxane elastomer,Polydimethylsiloxane tubing,Polyethylene T-body frame,Barium sulfate,Polypropylene removal threads.
Recently Viewed