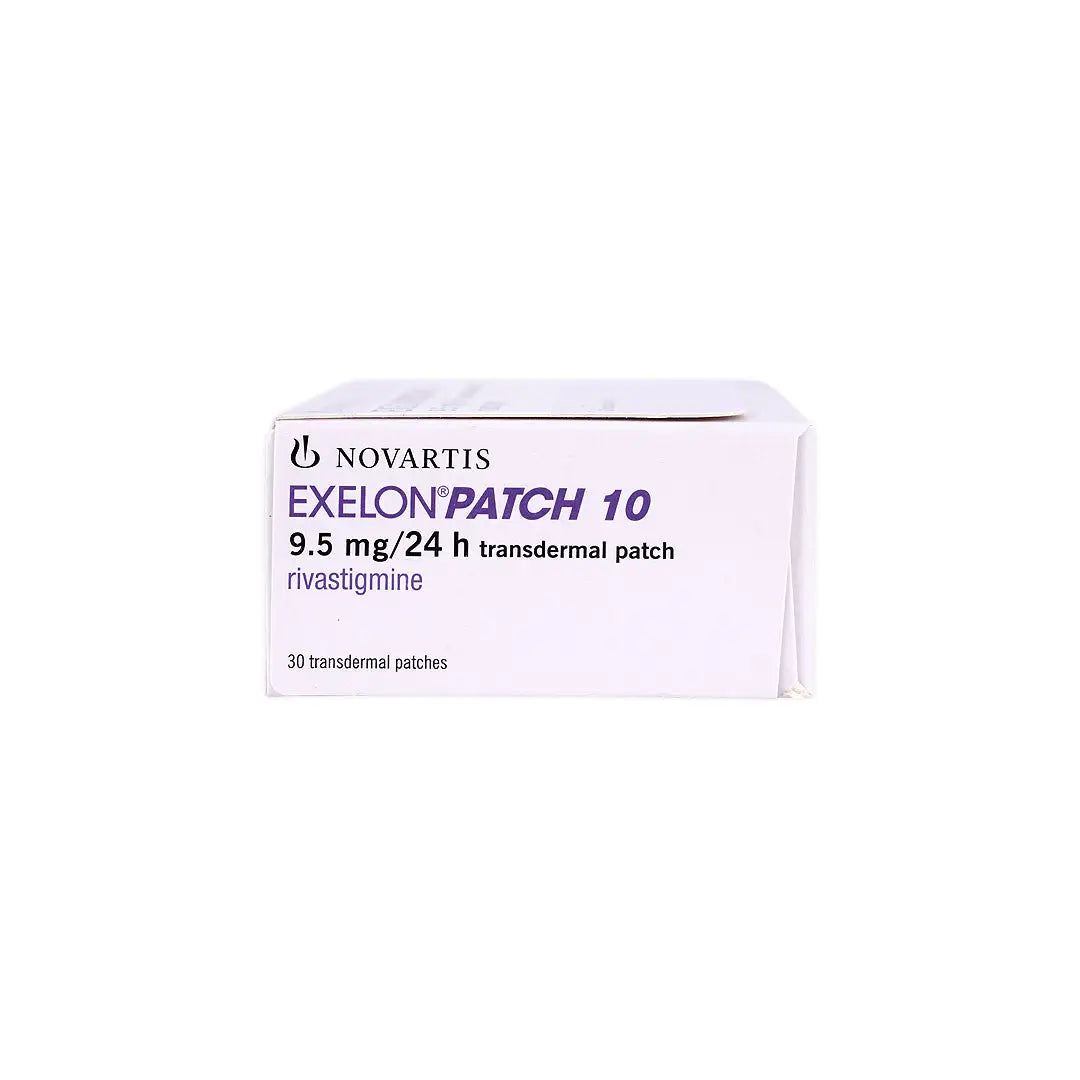
Novartis Pharma
SKU: 45761
A transdermal therapeutic system delivering rivastigmine, a cholinesterase inhibitor, used in the management of mild to moderately severe dementia associated with Alzheimer’s and Parkinson’s disease. The patch provides continuous drug release over 24 hours, ensuring steady plasma levels and improved tolerability compared to oral formulations.
Indications:
- Treatment of mild to moderately severe Alzheimer’s dementia.
- Treatment of mild to moderately severe dementia associated with Parkinson’s disease.
- Symptom management including memory loss, confusion, and behavioral changes.
Main Active Ingredient:
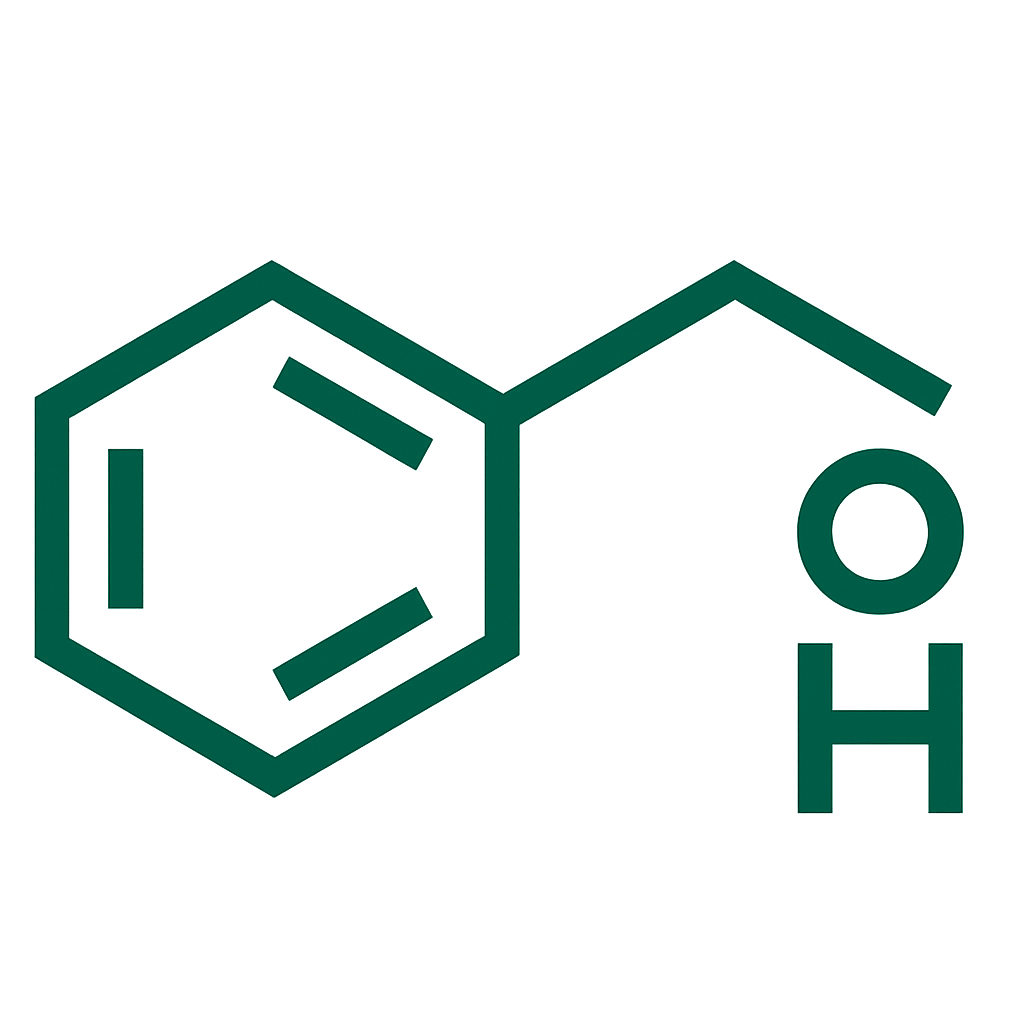
- Rivastigmine 9.5 mg per 24 hours (delivered via transdermal patch).
Precautions:
- For external transdermal use only .
- Avoid use if allergic to rivastigmine, other carbamate derivatives, or excipients.
- Use with caution in patients with:
- Low body weight (<50 kg).
- Gastrointestinal ulcers or active GI bleeding.
- Cardiac conduction abnormalities.
- History of seizures or asthma.
- Remove the previous patch before applying a new one.
- Rotate application sites to reduce skin irritation.
- Not recommended during pregnancy or breastfeeding unless clearly needed.
- Keep out of reach of children and pets.
Side Effects:
- Weight loss.
- Tremors.
- Insomnia.
- Abdominal pain.
- Anxiety.
- Urinary incontinence.
- Severe allergic reactions.
- Bradycardia.
- Syncope.
- Severe vomiting leading to dehydration.
- Gastrointestinal bleeding.
Drug–Drug Interactions:
- Increased risk of bradycardia with beta-blockers, digoxin, amiodarone, or other antiarrhythmics.
- Additive cholinergic effects with other cholinesterase inhibitors or cholinergic agonists.
- May antagonize the effects of anticholinergic drugs.
- Increased risk of gastrointestinal side effects when used with NSAIDs.
- Use with caution alongside neuromuscular blocking agents during anesthesia.
Indications:
- Treatment of mild to moderately severe Alzheimer’s dementia.
- Treatment of mild to moderately severe dementia associated with Parkinson’s disease.
- Symptom management including memory loss, confusion, and behavioral changes.
Main Active Ingredient:
- Rivastigmine 9.5 mg per 24 hours (delivered via transdermal patch).
Precautions:
- For external transdermal use only .
- Avoid use if allergic to rivastigmine, other carbamate derivatives, or excipients.
- Use with caution in patients with:
- Low body weight (<50 kg).
- Gastrointestinal ulcers or active GI bleeding.
- Cardiac conduction abnormalities.
- History of seizures or asthma.
- Remove the previous patch before applying a new one.
- Rotate application sites to reduce skin irritation.
- Not recommended during pregnancy or breastfeeding unless clearly needed.
- Keep out of reach of children and pets.
Side Effects:
- Weight loss.
- Tremors.
- Insomnia.
- Abdominal pain.
- Anxiety.
- Urinary incontinence.
- Severe allergic reactions.
- Bradycardia.
- Syncope.
- Severe vomiting leading to dehydration.
- Gastrointestinal bleeding.
Drug–Drug Interactions:
- Increased risk of bradycardia with beta-blockers, digoxin, amiodarone, or other antiarrhythmics.
- Additive cholinergic effects with other cholinesterase inhibitors or cholinergic agonists.
- May antagonize the effects of anticholinergic drugs.
- Increased risk of gastrointestinal side effects when used with NSAIDs.
- Use with caution alongside neuromuscular blocking agents during anesthesia.
Rivastigmine,Acrylic copolymer,Poly(butylmethacrylate, methacrylic acid),Poly(butylmethacrylate, 2-ethylhexylacrylate, vinyl acetate),Oleyl alcohol.
Recently Viewed