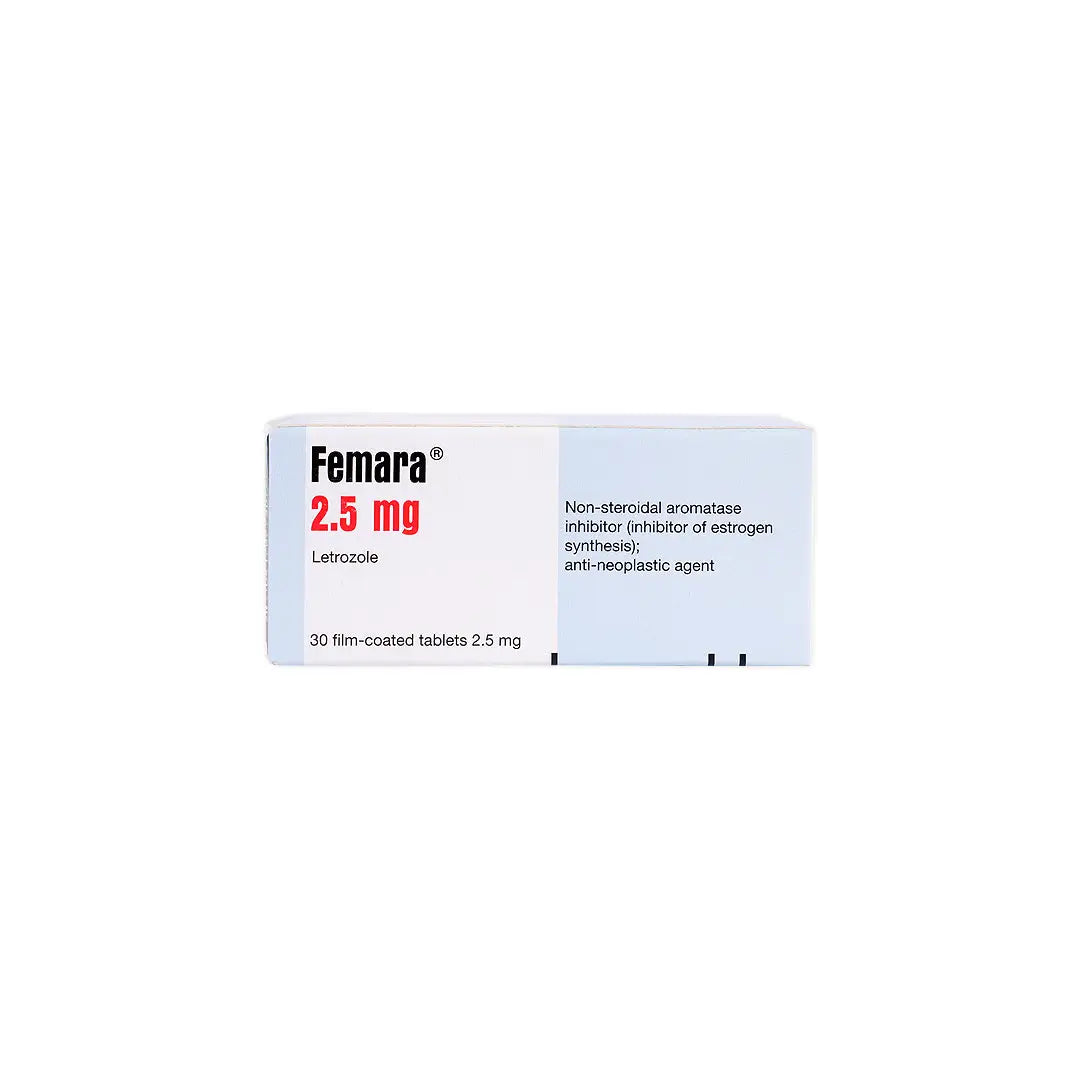
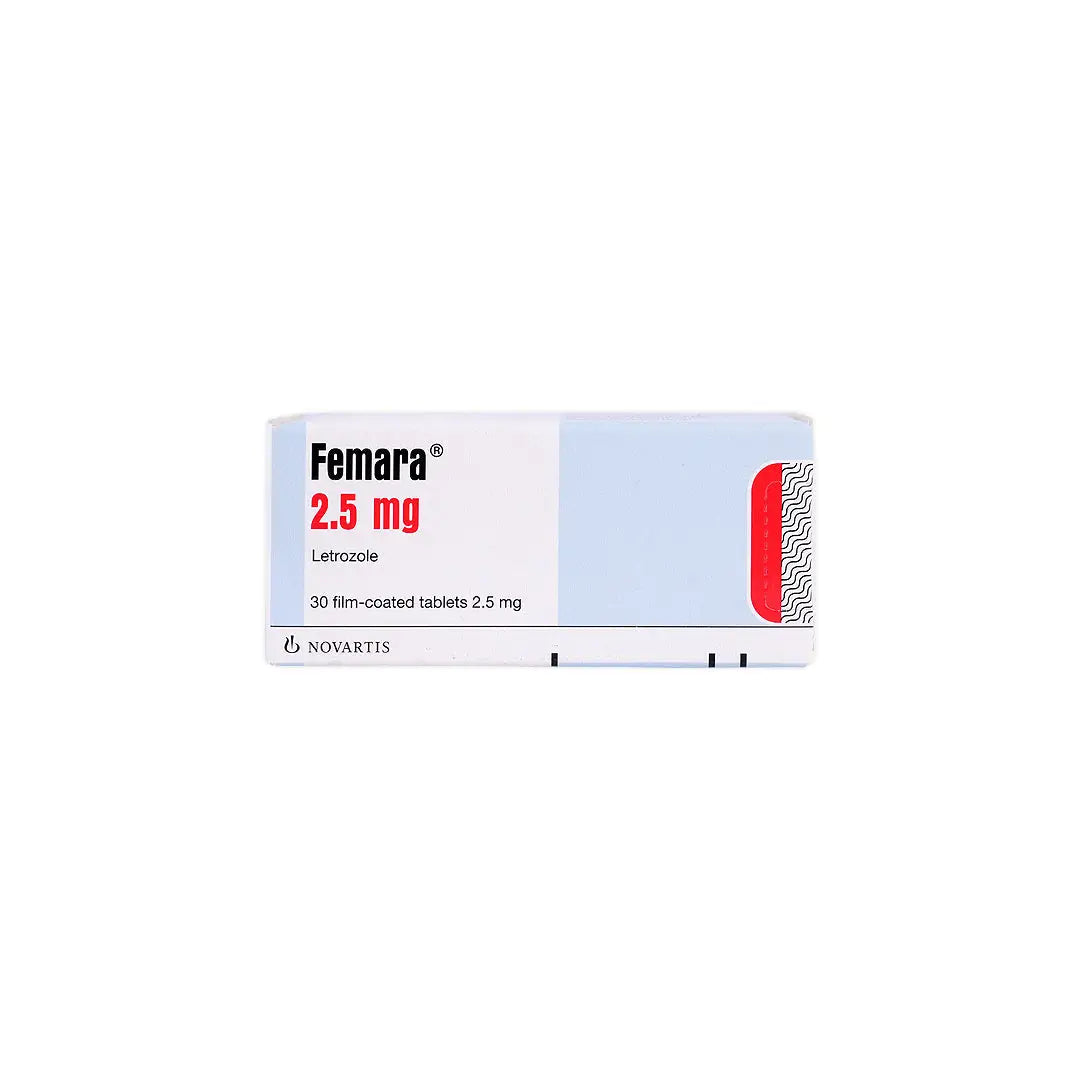
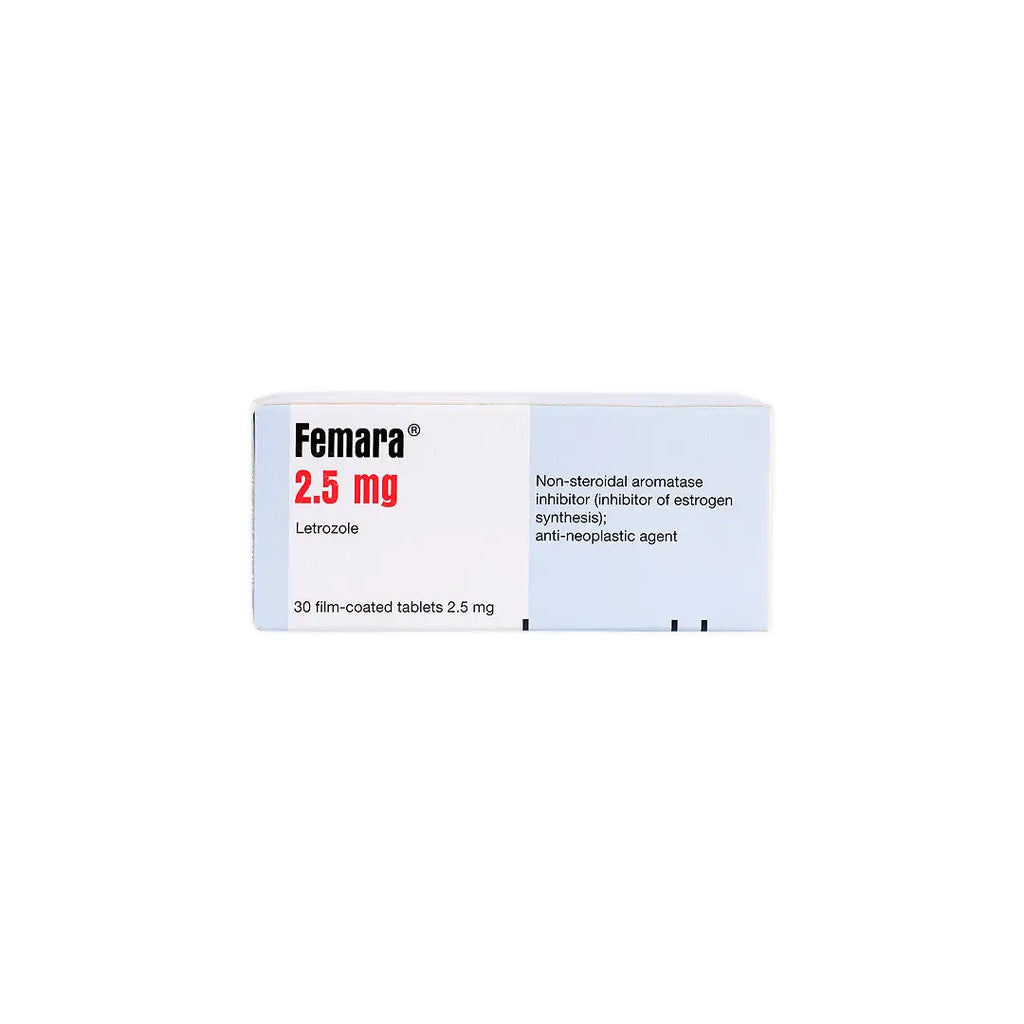
An oral aromatase inhibitor used to treat hormone receptor-positive breast cancer in postmenopausal women. It works by reducing estrogen production, thereby slowing or stopping the growth of estrogen-dependent cancer cells.
Indications:
- Adjuvant treatment of early-stage hormone receptor-positive breast cancer in postmenopausal women.
- Treatment of advanced or metastatic breast cancer in postmenopausal women.
- Extended therapy following 5 years of tamoxifen treatment.
- Management of breast cancer recurrence in postmenopausal patients.
Main Active Ingredient:
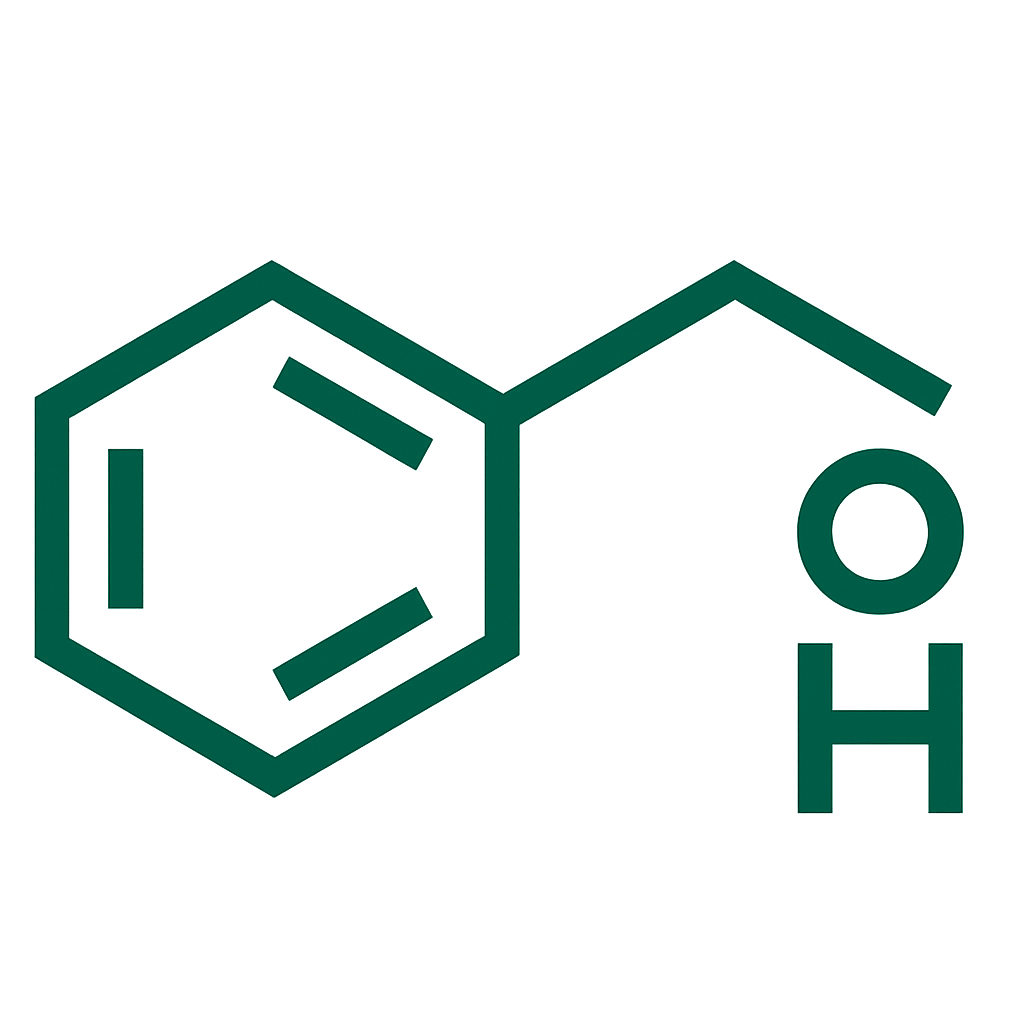
- Letrozole 2.5 mg per tablet.
Precautions:
- Not recommended for use in premenopausal women.
- Avoid during pregnancy or breastfeeding; effective contraception required for women of childbearing potential.
- Use caution in patients with:
- Severe liver impairment.
- Osteoporosis or low bone mineral density.
- Regular monitoring of liver function, lipid profile, and bone density is recommended.
- Inform your physician about any history of cardiovascular disease, fractures, or severe renal impairment.
Side Effects:
- Hot flashes.
- Fatigue.
- Joint pain
- Muscle pain
- Nausea.
- Headache.
- Increased sweating.
- Dizziness.
- Hair thinning.
- Insomnia.
- Edema.
- Increased fracture risk.
- Severe allergic reactions.
- Liver enzyme elevations.
- Cardiovascular events .
Drug–Drug Interactions:
- Caution with tamoxifen (antagonistic effects on estrogen receptors).
- May interact with drugs that strongly inhibit or induce CYP3A4, CYP2A6, or CYP2C19 enzymes, affecting letrozole metabolism.
- Concomitant use with estrogen-containing therapies (e.g., hormone replacement therapy) can reduce efficacy.
- No significant interactions reported with common medications like analgesics, antihypertensives, or statins.
Letrozole,Lactose monohydrate,Microcrystalline cellulose,Sodium starch glycolate,Povidone (polyvinylpyrrolidone),Magnesium stearate,Colloidal silicon dioxide.