SPIMACO
SKU: 49923
An extended-release oral antidiabetic medication used in the management of type 2 diabetes mellitus. It helps control blood glucose levels by reducing hepatic glucose production, decreasing intestinal absorption of glucose, and improving insulin sensitivity.
Indications:
- Management of type 2 diabetes mellitus (non-insulin-dependent diabetes).
- Can be used as monotherapy when diet and exercise alone are insufficient.
- May be used in combination with other antidiabetic agents (e.g., sulfonylureas, DPP-4 inhibitors, insulin) when - - additional glycemic control is required.
- Helps reduce the risk of diabetes-related complications when blood sugar is controlled.
Main Active Ingredient::
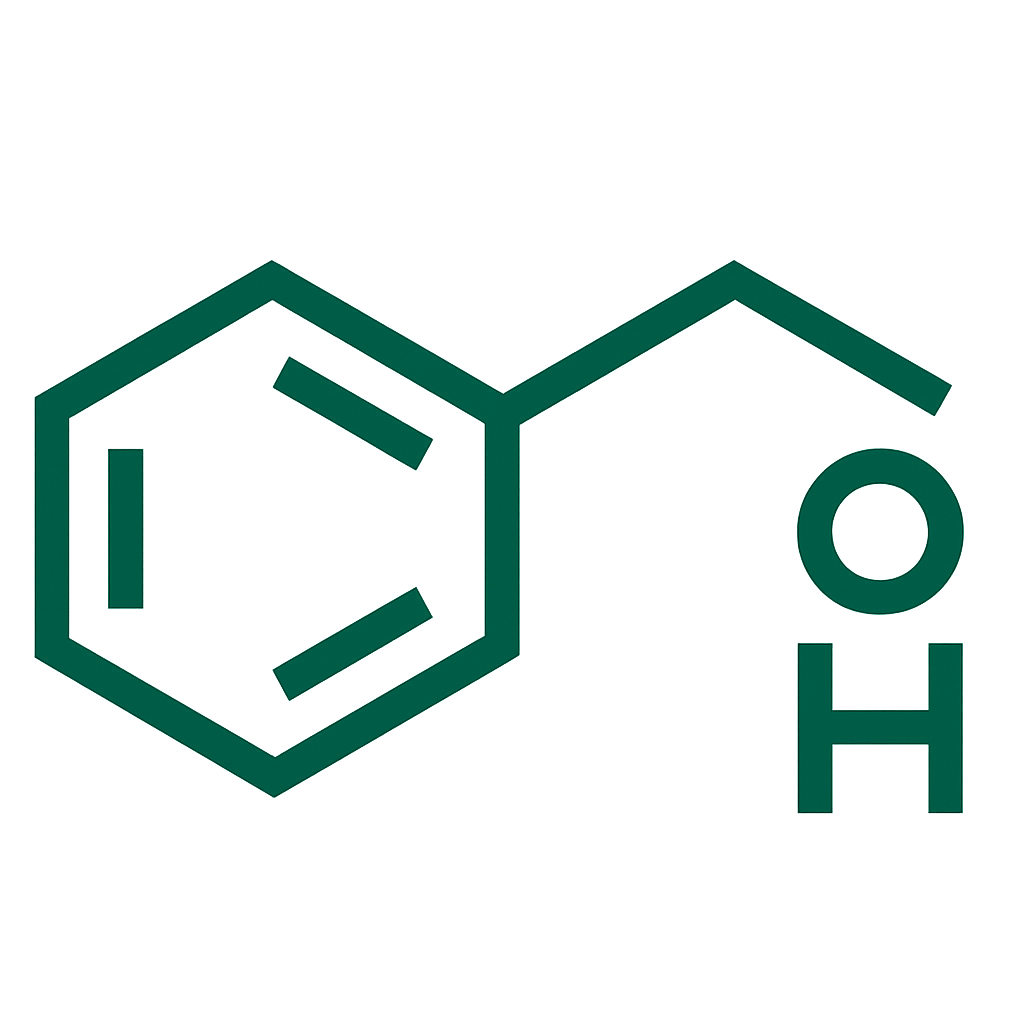
- Metformin Hydrochloride (Extended Release) 750 mg per tablet.
Precautions:
- For oral use only.
- Contraindicated in patients with:
- Severe renal impairment (eGFR < 30 mL/min/1.73m²).
- Acute or chronic metabolic acidosis (including diabetic ketoacidosis).
- Severe liver disease or alcoholism.
Use with caution in:
- Elderly patients.
- Those with heart failure, hypoxia, or dehydration.
- Patients undergoing surgery or radiological procedures with iodinated contrast (risk of lactic acidosis).
- Avoid excessive alcohol intake as it increases the risk of lactic acidosis.
- Regular monitoring of kidney and liver function is advised.
- Not recommended during pregnancy or breastfeeding unless prescribed.
Side Effects:
- Nausea.
- Vomiting.
- Diarrhea.
- Abdominal pain.
- loss of appetite.
- Taste disturbances.
- Headache.
- Mild weight loss.
- Lactic acidosis.
- Long-term use May reduce vitamin B12 absorption, leading to deficiency.
Drug–Drug Interactions:
- Iodinated contrast media.
- Alcohol.
- Cimetidine and other drugs affecting renal clearance.
- Diuretics.
- Certain antihypertensives (ACE inhibitors, ARBs).
Indications:
- Management of type 2 diabetes mellitus (non-insulin-dependent diabetes).
- Can be used as monotherapy when diet and exercise alone are insufficient.
- May be used in combination with other antidiabetic agents (e.g., sulfonylureas, DPP-4 inhibitors, insulin) when - - additional glycemic control is required.
- Helps reduce the risk of diabetes-related complications when blood sugar is controlled.
Main Active Ingredient::
- Metformin Hydrochloride (Extended Release) 750 mg per tablet.
Precautions:
- For oral use only.
- Contraindicated in patients with:
- Severe renal impairment (eGFR < 30 mL/min/1.73m²).
- Acute or chronic metabolic acidosis (including diabetic ketoacidosis).
- Severe liver disease or alcoholism.
Use with caution in:
- Elderly patients.
- Those with heart failure, hypoxia, or dehydration.
- Patients undergoing surgery or radiological procedures with iodinated contrast (risk of lactic acidosis).
- Avoid excessive alcohol intake as it increases the risk of lactic acidosis.
- Regular monitoring of kidney and liver function is advised.
- Not recommended during pregnancy or breastfeeding unless prescribed.
Side Effects:
- Nausea.
- Vomiting.
- Diarrhea.
- Abdominal pain.
- loss of appetite.
- Taste disturbances.
- Headache.
- Mild weight loss.
- Lactic acidosis.
- Long-term use May reduce vitamin B12 absorption, leading to deficiency.
Drug–Drug Interactions:
- Iodinated contrast media.
- Alcohol.
- Cimetidine and other drugs affecting renal clearance.
- Diuretics.
- Certain antihypertensives (ACE inhibitors, ARBs).
metformin hydrochloride
Recently Viewed