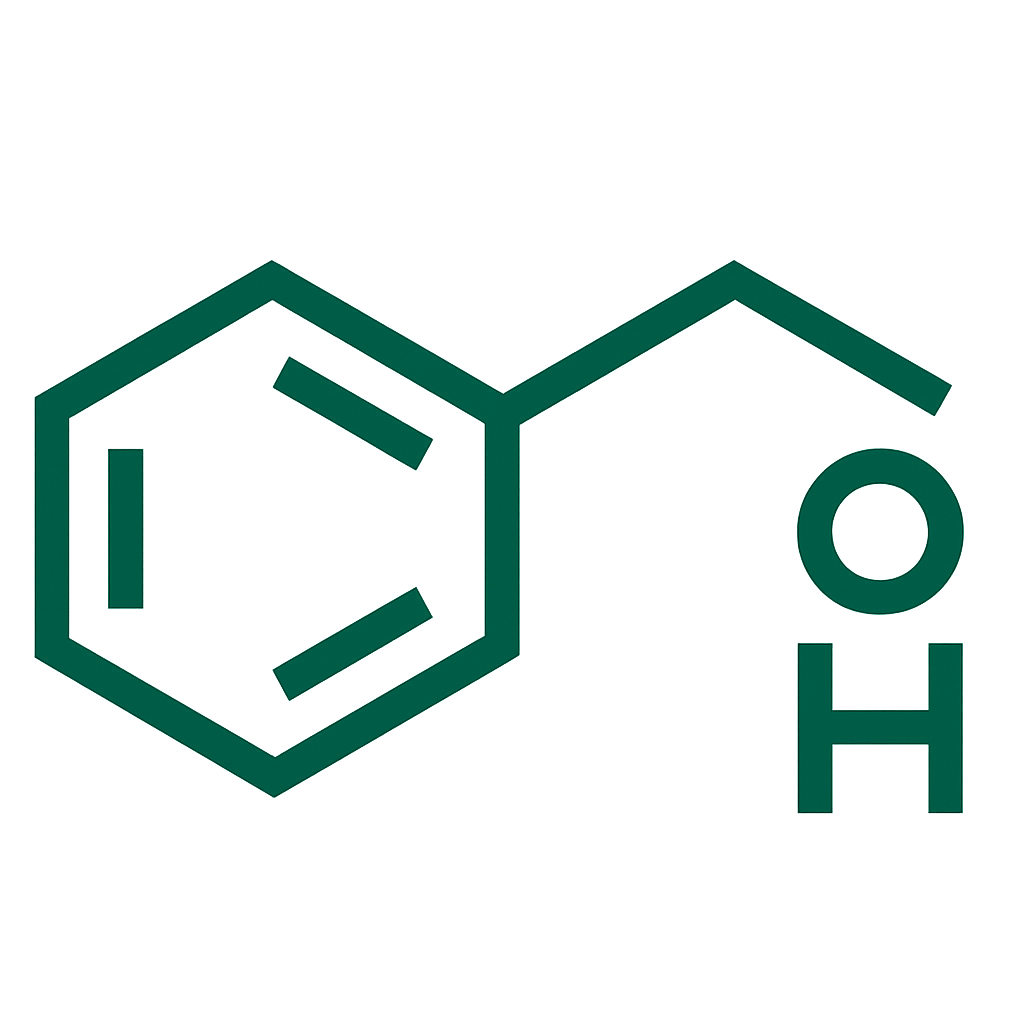
A 4 mg / 2 mL injectable/infusion formulation of ondansetron used to prevent and treat nausea and vomiting in various settings by antagonizing serotonin (5-HT₃) receptors.
Indications:
Prevention of nausea and vomiting caused by cancer chemotherapy.
Postoperative nausea and vomiting.
Radiotherapy induced nausea and vomiting.
Main Active Ingredient:
- Ondansetron, 4 mg per 2 mL ampoule.
Precautions:
- Should be given according to medical prescription only.
- Monitor patients with existing QT prolongation or cardiac conduction abnormalities.
- Use caution in patients with hepatic impairment; dose adjustments may be needed.
- Pregnancy & breastfeeding: use only if clearly needed. Consult physician.
- Ensure correct route of administration and avoid extravasation.
- Avoid use in patients allergic to ondansetron or its excipients.
Side Effects:
- Headache.
- Constipation.
- Diarrhea.
- Dizziness or light-headedness.
- Fatigue, malaise.
- QT interval prolongation.
- Hypersensitivity reactions (rash, itching, swelling).
- Liver function changes in rare cases.
Drug-Drug Interactions:
- Other drugs that prolong QT interval (e.g., some antiarrhythmics, certain antipsychotics, some antibiotics) increase risk of cardiac arrhythmias when used with ondansetron.
- Drugs that inhibit or induce hepatic enzymes metabolizing ondansetron may affect its levels and effects.
- Concurrent use of other serotonin-modulating agents may increase risk of serotonin syndrome.
- Administer via intravenous injection or infusion as per physician order.
- Dose timing: give before the emetogenic stimulus as directed.
- Follow up doses may be required depending on treatment.
- Ensure correct dilution and infusion rate if applicable.
- Monitor patient during and after administration for adverse reactions.
Ondansetron hydrochloride dihydrate,Sodium chloride,Citric acid monohydrate,Sodium citrate dihydrate,Water for injections.