Lg Life Science Ltd.Korea
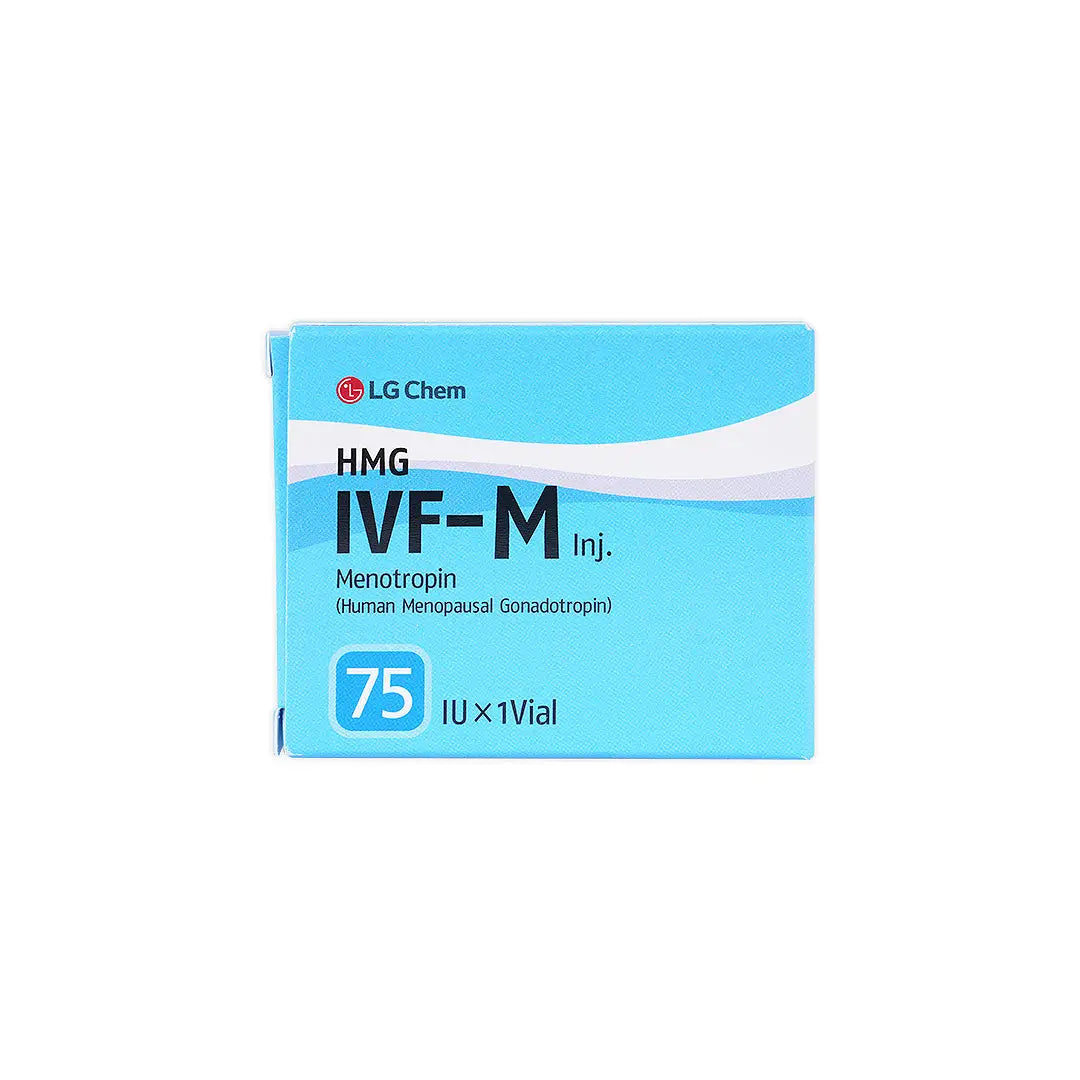
SKU: 21503
A gonadotropin preparation containing highly purified human menopausal gonadotropin (hMG), which has both follicle-stimulating hormone (FSH) and luteinizing hormone (LH) activity. It is used in fertility treatment programs to stimulate the ovaries in women and to support spermatogenesis in men.
Indications:
- Stimulation of follicular development in women undergoing assisted reproductive technologies (ART) such as IVF.
- Treatment of anovulation, including polycystic ovary syndrome (PCOS), in women unresponsive to clomiphene citrate.
- Stimulation of spermatogenesis in men with primary or secondary hypogonadotropic hypogonadism (with hCG).
- Controlled ovarian hyperstimulation in infertility treatment programs.
Main Active Ingredient:
- Human Menopausal Gonadotropin (hMG).
Precautions:
- To be used only under the supervision of a fertility specialist.
- Contraindicated in patients with ovarian, testicular, or breast tumors.
- Not to be used in women with ovarian cysts not due to PCOS.
- Caution in patients with thyroid or adrenal disorders (must be treated first).
- Risk of ovarian hyperstimulation syndrome (OHSS) in women.
- Discontinue if ovarian enlargement or severe abdominal pain occurs.
- Multiple pregnancy and ectopic pregnancy risks must be considered.
- Use cautiously in patients with thrombophilia or risk of blood clots.
Possible Side Effects:
- Headache.
- Abdominal bloating.
- Pelvic discomfort.
- Injection site reactions.
- Ovarian hyperstimulation syndrome (OHSS) .
- Nausea.
- Vomiting.
- Breast tenderness.
- Multiple pregnancy.
- In men: gynecomastia.
- Acne.
- Fluid retention.
- Allergic reactions.
Drug-Drug Interactions:
- Clomiphene citrate.
- Other gonadotropins (FSH, hCG).
- GnRH agonists/antagonists.
- No major systemic drug interactions reported, but should be administered only in specialist fertility protocols.
Indications:
- Stimulation of follicular development in women undergoing assisted reproductive technologies (ART) such as IVF.
- Treatment of anovulation, including polycystic ovary syndrome (PCOS), in women unresponsive to clomiphene citrate.
- Stimulation of spermatogenesis in men with primary or secondary hypogonadotropic hypogonadism (with hCG).
- Controlled ovarian hyperstimulation in infertility treatment programs.
Main Active Ingredient:
- Human Menopausal Gonadotropin (hMG).
Precautions:
- To be used only under the supervision of a fertility specialist.
- Contraindicated in patients with ovarian, testicular, or breast tumors.
- Not to be used in women with ovarian cysts not due to PCOS.
- Caution in patients with thyroid or adrenal disorders (must be treated first).
- Risk of ovarian hyperstimulation syndrome (OHSS) in women.
- Discontinue if ovarian enlargement or severe abdominal pain occurs.
- Multiple pregnancy and ectopic pregnancy risks must be considered.
- Use cautiously in patients with thrombophilia or risk of blood clots.
Possible Side Effects:
- Headache.
- Abdominal bloating.
- Pelvic discomfort.
- Injection site reactions.
- Ovarian hyperstimulation syndrome (OHSS) .
- Nausea.
- Vomiting.
- Breast tenderness.
- Multiple pregnancy.
- In men: gynecomastia.
- Acne.
- Fluid retention.
- Allergic reactions.
Drug-Drug Interactions:
- Clomiphene citrate.
- Other gonadotropins (FSH, hCG).
- GnRH agonists/antagonists.
- No major systemic drug interactions reported, but should be administered only in specialist fertility protocols.
Human Menopausal Gonadotropin (hMG),Lactose monohydrate,Polysorbate 20,
Recently Viewed